Menu
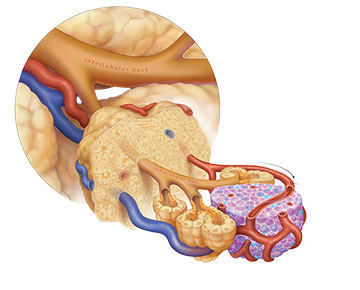
Advances towards a cure for diabetes are gaining significant traction with researchers now harnessing the power of stem cells to create functional insulin-producing beta cells that can replace the damaged ones in patients with diabetes. This therapy has the potential to allow diabetic patients to forgo daily insulin injections and provide them with biological cure to allow them to physiologically control their blood sugar levels.
However, the next hurdle that needs to be addressed is where and how to deliver these cells in the human body and then the ability to track their function over time. Our work in IRIS is focused on creating novel bioengineered delivery devices through advanced 3D printing techniques, technologies that can optimize the site of islet implantation, and novel imaging techniques to monitor transplanted islets. IRIS is also connected to the allogenic and autologous islet transplantation programs at Stanford as well as the Stanford Diabetes Research Center to ensure that innovative solutions can be effectively translated into patients.

Vascular stents play a crucial role in maintaining the integrity and patency of injured or compromised blood vessels, ensuring essential blood flow to tissues and organs. However, traditional stents are typically permanent solutions, which can pose significant challenges, especially for pediatric patients. As children grow, these fixed-size stents can become inadequate, leading to complications that may require additional interventions later in life. IRIS is revolutionizing this field with an innovative biodegradable stent technology. Designed to provide temporary support during the healing and remodeling of blood vessels, these stents act like a crutch for the body, offering stability while allowing for natural recovery. Over time, the stent gradually dissolves, eliminating the need for a permanent implant and reducing the risk of long-term complications.
Leveraging advancements in 3D printing, IRIS is creating stents with previously unattainable geometries and attributes, including the ability to grow alongside the patient. This means that each stent can be custom printed to fit the unique anatomy of individual patients, providing a truly personalized solution that adapts to their changing needs. This innovative approach represents a significant leap forward in pediatric vascular care, ensuring that children can thrive without the burden of permanent implants. Furthermore, the ability of these biodegradable stents to be functionalized with biological therapies allows them to enhance healing and promote vascular regeneration. By incorporating bioactive agents, such as growth factors or anti-inflammatory compounds, directly into the stent material, these devices can actively contribute to the healing process while providing structural support. This dual functionality not only aids in the immediate recovery of the vessel but also encourages the natural remodeling of the tissue, potentially reducing the risk of complications such as restenosis. The release of these therapeutic agents can be precisely controlled, allowing for a tailored approach to treatment that aligns with the specific needs of each patient.

Neuromodulation endovascular devices are innovative tools designed to target specific nerves in the brain that can control bodily functions and hence possess significant potential for recovery after stroke. These minimally invasive devices are delivered through blood vessels into the brain, where they can either send gentle electrical signals to nerves. By influencing how these nerves work, the devices can help control the body through external computer interfaces. Because their implantation is less invasive than surgery, development of these devices can offer patients quicker recovery times and fewer complications.

When delivering therapies to organs, it is crucial for these treatments to navigate various tissue barriers to reach their intended target cells. This journey begins at cellular interfaces, such as endothelial cells for therapies delivered through the bloodstream and epithelial cells for those introduced into biological lumens. From there, therapies must traverse tissue matrices made up of the extracellular matrix (ECM), which consists of collagen and glycoproteins, as well as the interstitial space regulated by the lymphatic system. Understanding these barriers is vital, especially since certain organs have specialized protective layers, like the blood-brain barrier (BBB) in the brain, the glomeruli in the kidneys, and the placenta during pregnancy. Additionally, diseases can alter how these barriers function; for example, some tumors may develop increased stroma and high interstitial pressures that hinder therapy access, while inflammatory conditions can lead to dysfunctional or leaky barriers that make it difficult for therapies to remain effective.
For many years, researchers have recognized that modifying the tissue microenvironment can enhance therapy delivery. This challenge has spurred the development of innovative technologies that can precisely and temporarily alter tissue microenvironments. In IRIS, we are developing technologies like focused ultrasound (FUS), which we have shown can increase vessel permeability, stimulate regenerative pathways and encourage stem cell therapy homing and retention.